Insulinomas are rare pancreatic tumors that secrete excessive insulin, leading to hypoglycemia, while glucagonomas produce excess glucagon, causing hyperglycemia and characteristic skin rash called necrolytic migratory erythema. Both tumors are types of pancreatic neuroendocrine tumors but differ significantly in hormonal effects and clinical presentation. Accurate diagnosis through biochemical tests and imaging is crucial for effective treatment and management.
Table of Comparison
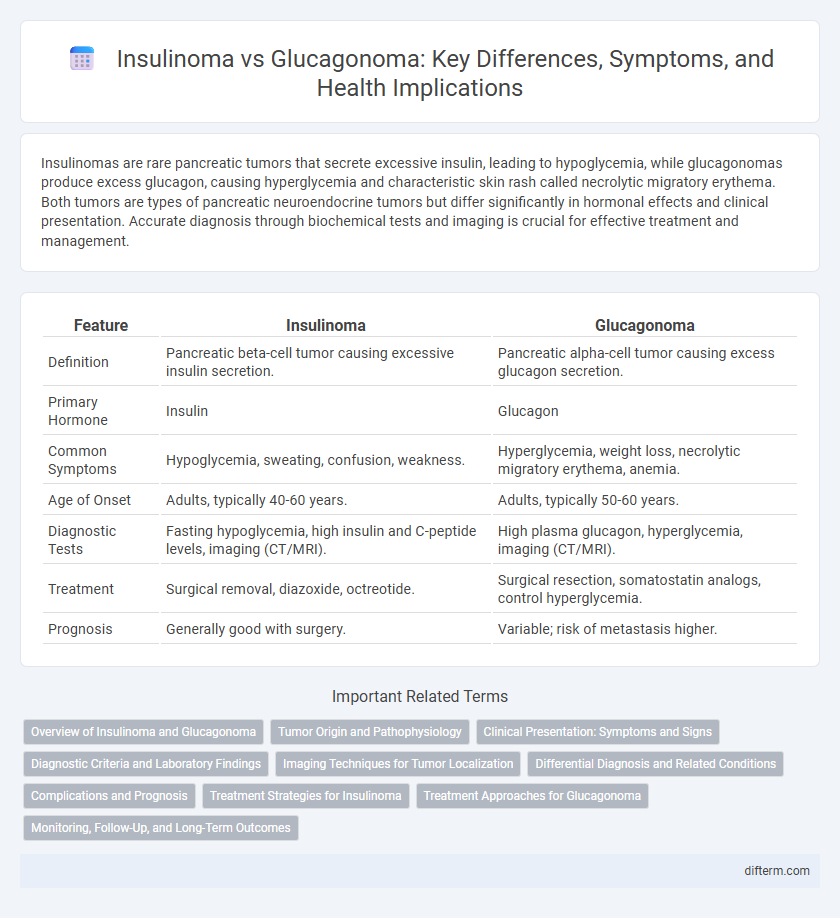
| Feature | Insulinoma | Glucagonoma |
|---|---|---|
| Definition | Pancreatic beta-cell tumor causing excessive insulin secretion. | Pancreatic alpha-cell tumor causing excess glucagon secretion. |
| Primary Hormone | Insulin | Glucagon |
| Common Symptoms | Hypoglycemia, sweating, confusion, weakness. | Hyperglycemia, weight loss, necrolytic migratory erythema, anemia. |
| Age of Onset | Adults, typically 40-60 years. | Adults, typically 50-60 years. |
| Diagnostic Tests | Fasting hypoglycemia, high insulin and C-peptide levels, imaging (CT/MRI). | High plasma glucagon, hyperglycemia, imaging (CT/MRI). |
| Treatment | Surgical removal, diazoxide, octreotide. | Surgical resection, somatostatin analogs, control hyperglycemia. |
| Prognosis | Generally good with surgery. | Variable; risk of metastasis higher. |
Overview of Insulinoma and Glucagonoma
Insulinoma and glucagonoma are rare pancreatic neuroendocrine tumors with distinct hormonal secretions driving their clinical features. Insulinomas primarily secrete insulin, leading to hypoglycemia with symptoms such as confusion, sweating, and palpitations, while glucagonomas overproduce glucagon, causing hyperglycemia, weight loss, and characteristic necrolytic migratory erythema. Diagnosis often involves biochemical tests measuring hormone levels and imaging studies like endoscopic ultrasound or CT scans to localize the tumors.
Tumor Origin and Pathophysiology
Insulinomas originate from pancreatic beta cells and cause excessive insulin secretion, leading to hypoglycemia due to increased glucose uptake by tissues. Glucagonomas arise from pancreatic alpha cells and result in elevated glucagon levels, causing hyperglycemia and a catabolic state by promoting gluconeogenesis and glycogenolysis. Both tumors disrupt normal glucose homeostasis but through opposing hormonal imbalances derived from distinct pancreatic islet cells.
Clinical Presentation: Symptoms and Signs
Insulinomas typically present with symptoms of hypoglycemia such as sweating, confusion, tremors, and palpitations due to excessive insulin secretion. Glucagonomas manifest with hyperglycemia, weight loss, and characteristic necrolytic migratory erythema, alongside glossitis and anemia. Both tumors are rare pancreatic neuroendocrine neoplasms but exhibit contrasting clinical presentations driven by their respective hormone secretions.
Diagnostic Criteria and Laboratory Findings
Insulinoma diagnosis relies on the Whipple triad, confirmed by supervised 72-hour fasting test showing inappropriately high insulin levels (>3 mU/mL) with hypoglycemia (glucose <55 mg/dL) and elevated C-peptide indicating endogenous insulin production. Glucagonoma diagnosis involves elevated fasting plasma glucagon levels (>500 pg/mL) alongside symptoms of hyperglycemia, weight loss, and necrolytic migratory erythema, confirmed by imaging studies detecting pancreatic alpha-cell tumors. Laboratory differentiation between insulinoma and glucagonoma centers on hormone quantification and corresponding glycemic profiles, critical for targeted treatment planning.
Imaging Techniques for Tumor Localization
Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) scans are primary imaging techniques utilized for localizing insulinomas and glucagonomas, offering high-resolution visualization of pancreatic tumors. Endoscopic ultrasound (EUS) provides superior sensitivity for detecting small insulinomas, often less than 2 cm, by enabling close proximity imaging and fine-needle aspiration for cytological confirmation. Somatostatin receptor scintigraphy (SRS) and positron emission tomography (PET) with radiolabeled somatostatin analogs improve localization accuracy for glucagonomas, which typically express somatostatin receptors, aiding in precise tumor mapping and staging.
Differential Diagnosis and Related Conditions
Insulinoma and glucagonoma are pancreatic neuroendocrine tumors distinguished by their hormone secretion profiles, with insulinoma causing hypoglycemia due to excessive insulin release, while glucagonoma leads to hyperglycemia and characteristic necrolytic migratory erythema from glucagon hypersecretion. Differential diagnosis involves measuring serum insulin, C-peptide, and glucose levels during symptomatic episodes, alongside assessing plasma glucagon concentrations and imaging studies such as endoscopic ultrasound or MRI to localize the tumor. Related conditions include multiple endocrine neoplasia type 1 (MEN1), which predisposes patients to both insulinoma and glucagonoma along with other endocrine tumors.
Complications and Prognosis
Insulinoma primarily leads to hypoglycemia-related complications such as neuroglycopenic symptoms and seizures, while glucagonoma causes hyperglycemia and characteristic skin rash called necrolytic migratory erythema, along with weight loss and anemia. Insulinomas generally have a favorable prognosis with surgical resection, whereas glucagonomas, often malignant at diagnosis, carry a poorer prognosis due to metastatic potential and complications like diabetes mellitus and thromboembolism. Early diagnosis and appropriate management significantly influence outcomes for both pancreatic neuroendocrine tumors.
Treatment Strategies for Insulinoma
Surgical resection remains the primary treatment strategy for insulinoma, often resulting in a high cure rate due to the tumor's typically benign nature. Medical management with diazoxide and octreotide is used to control hypoglycemia in patients who are not surgical candidates or have metastatic disease. Emerging therapies include targeted molecular treatments and peptide receptor radionuclide therapy, which are being investigated to improve outcomes in malignant or recurrent insulinomas.
Treatment Approaches for Glucagonoma
Glucagonoma treatment primarily involves surgical resection of the tumor to achieve potential cure, particularly when detected early and localized. Medical management includes the use of somatostatin analogs like octreotide to control glucagon secretion and alleviate symptoms such as necrolytic migratory erythema and hyperglycemia. Chemotherapy and targeted therapies may be considered for metastatic or inoperable glucagonomas to improve patient outcomes.
Monitoring, Follow-Up, and Long-Term Outcomes
Insulinoma patients require frequent blood glucose monitoring to detect hypoglycemic episodes and regular imaging studies such as MRI or CT scans for tumor surveillance. Glucagonoma management involves monitoring glucagon levels and nutritional status, alongside periodic imaging to assess tumor progression and metastasis. Long-term outcomes for insulinoma are generally favorable with surgical resection, while glucagonoma often has a more guarded prognosis due to frequent malignancy and recurrence risk.
Insulinoma vs Glucagonoma Infographic
 difterm.com
difterm.com