Case-control studies compare individuals with a specific condition to those without, identifying factors that may contribute to the disease by looking retrospectively. Cohort studies follow a group over time to observe how exposure to certain risk factors influences the development of health outcomes, allowing for assessment of incidence and temporal relationships. Both designs are essential in epidemiology, with case-control studies being more efficient for rare diseases and cohort studies providing stronger evidence for causal inference.
Table of Comparison
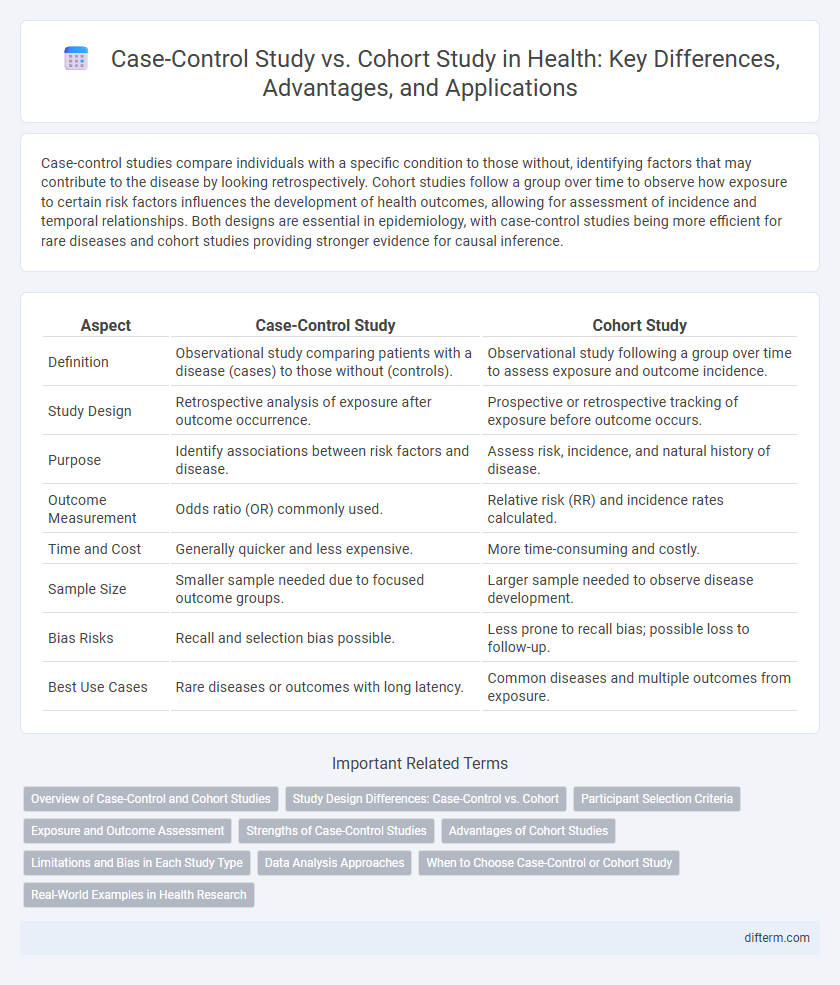
| Aspect | Case-Control Study | Cohort Study |
|---|---|---|
| Definition | Observational study comparing patients with a disease (cases) to those without (controls). | Observational study following a group over time to assess exposure and outcome incidence. |
| Study Design | Retrospective analysis of exposure after outcome occurrence. | Prospective or retrospective tracking of exposure before outcome occurs. |
| Purpose | Identify associations between risk factors and disease. | Assess risk, incidence, and natural history of disease. |
| Outcome Measurement | Odds ratio (OR) commonly used. | Relative risk (RR) and incidence rates calculated. |
| Time and Cost | Generally quicker and less expensive. | More time-consuming and costly. |
| Sample Size | Smaller sample needed due to focused outcome groups. | Larger sample needed to observe disease development. |
| Bias Risks | Recall and selection bias possible. | Less prone to recall bias; possible loss to follow-up. |
| Best Use Cases | Rare diseases or outcomes with long latency. | Common diseases and multiple outcomes from exposure. |
Overview of Case-Control and Cohort Studies
Case-control studies compare individuals with a specific condition (cases) to those without (controls) to identify factors that may contribute to the condition, making them efficient for studying rare diseases. Cohort studies follow a group of people over time to observe how exposures affect the incidence of outcomes, providing strong evidence for causal relationships. Both study designs are fundamental in epidemiology but differ in structure, timeline, and applications for assessing disease risk factors.
Study Design Differences: Case-Control vs. Cohort
Case-control studies retrospectively compare individuals with a specific disease (cases) to those without (controls) to identify factors linked to the disease, offering efficiency for rare conditions. Cohort studies prospectively follow groups with varying exposure levels over time to observe disease incidence, enabling direct risk estimation and temporal relationship assessment. The primary design difference lies in directionality: case-control studies start with outcomes and look backward, while cohort studies begin with exposures and track forward.
Participant Selection Criteria
Case-control studies select participants based on disease status, comparing individuals with the condition (cases) to those without (controls) to investigate prior exposures. Cohort studies enroll participants based on exposure status, following exposed and unexposed groups over time to observe disease incidence. The selection criteria in case-control studies prioritize outcome presence at the start, while cohort studies emphasize initial exposure status for temporal relationship assessment.
Exposure and Outcome Assessment
Case-control studies are retrospective and start by identifying individuals based on outcome status, then assessing past exposure through interviews or records, which may introduce recall bias. Cohort studies are prospective or retrospective, selecting participants based on exposure status and following them over time to observe outcome development, allowing clearer temporal relationships. Exposure in cohort studies is measured before outcome occurrence, enhancing causal inference, whereas case-control studies rely on assessing exposure after outcome, potentially affecting accuracy.
Strengths of Case-Control Studies
Case-control studies offer significant strengths in epidemiological research, particularly their efficiency in investigating rare diseases and conditions with long latency periods. These studies require fewer resources and less time compared to cohort studies because they begin with cases and controls, allowing for quicker identification of potential risk factors. Moreover, case-control designs facilitate the assessment of multiple exposures related to a single outcome, providing valuable insights into disease etiology.
Advantages of Cohort Studies
Cohort studies provide the advantage of measuring incidence rates and establishing temporal sequences between exposures and outcomes, which is critical for understanding causality in health research. They allow researchers to study multiple outcomes from a single exposure, increasing the efficiency of data collection on disease development over time. Additionally, cohort studies reduce recall bias by prospectively collecting exposure data before disease onset, enhancing the reliability of health risk assessments.
Limitations and Bias in Each Study Type
Case-control studies often face recall bias and selection bias since participants are chosen based on disease status, potentially affecting exposure assessment accuracy. Cohort studies, while minimizing recall bias by following groups over time, can suffer from loss to follow-up bias and may require extensive resources, impacting data completeness. Both study types must address confounding variables to reduce bias and improve the validity of causal inferences in health research.
Data Analysis Approaches
Case-control studies analyze data by comparing exposure histories between patients with the disease (cases) and those without (controls), often using odds ratios to estimate risk. Cohort studies follow exposed and unexposed groups over time, employing incidence rates and relative risk calculations to assess the association between exposure and outcome. Both study designs utilize multivariate regression models to adjust for confounding variables and strengthen causal inference in epidemiological research.
When to Choose Case-Control or Cohort Study
Case-control studies are ideal for investigating rare diseases or outcomes by comparing individuals with the condition to those without, enabling efficient analysis of multiple risk factors. Cohort studies suit scenarios requiring observation of incidence and natural disease progression over time, tracking exposure to risk factors before outcomes occur. Choosing between these designs depends on factors such as disease rarity, study duration, resource availability, and the need to establish temporal sequences between exposure and outcome.
Real-World Examples in Health Research
Case-control studies, such as the investigation of smoking and lung cancer risk, effectively identify associations by comparing patients with the disease to those without, enabling quick insights into rare conditions. Cohort studies, exemplified by the Framingham Heart Study, track large populations over time to evaluate the impact of risk factors on disease development, providing comprehensive data on causality and temporal relationships. Both designs are essential in health research, with case-control studies offering efficiency for rare diseases and cohort studies delivering robust evidence through long-term observation.
Case-control study vs cohort study Infographic
 difterm.com
difterm.com