Bioavailability refers to the extent and rate at which a drug or nutrient is absorbed and becomes available at the site of physiological activity, directly affecting its therapeutic effectiveness. Bioequivalence indicates that two pharmaceutical products release their active ingredient into the bloodstream at the same rate and extent, ensuring consistent clinical performance. Understanding the distinction between bioavailability and bioequivalence is crucial for optimizing drug formulation and ensuring patient safety in health treatments.
Table of Comparison
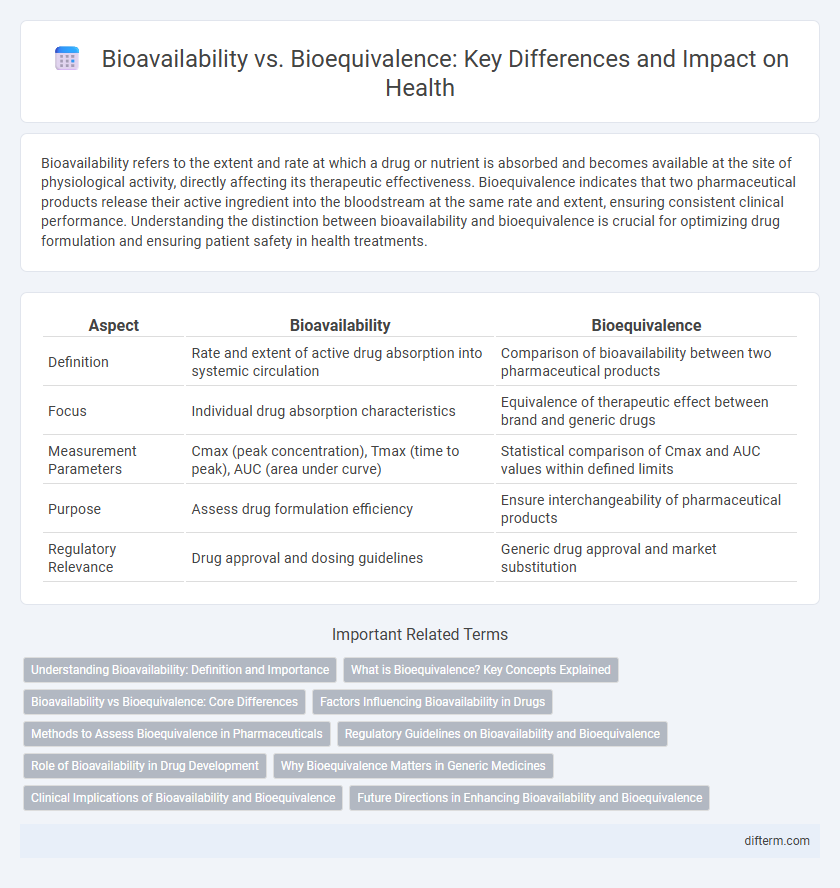
| Aspect | Bioavailability | Bioequivalence |
|---|---|---|
| Definition | Rate and extent of active drug absorption into systemic circulation | Comparison of bioavailability between two pharmaceutical products |
| Focus | Individual drug absorption characteristics | Equivalence of therapeutic effect between brand and generic drugs |
| Measurement Parameters | Cmax (peak concentration), Tmax (time to peak), AUC (area under curve) | Statistical comparison of Cmax and AUC values within defined limits |
| Purpose | Assess drug formulation efficiency | Ensure interchangeability of pharmaceutical products |
| Regulatory Relevance | Drug approval and dosing guidelines | Generic drug approval and market substitution |
Understanding Bioavailability: Definition and Importance
Bioavailability refers to the proportion of a drug or nutrient that enters the bloodstream and is available for therapeutic effect after administration. It is a critical factor in determining the efficacy and dosage requirements of medications, influencing how quickly and efficiently the active ingredients are absorbed. Understanding bioavailability ensures accurate drug formulation and personalized treatment plans, optimizing health outcomes.
What is Bioequivalence? Key Concepts Explained
Bioequivalence refers to the comparison between two pharmaceutical products to ensure they deliver the same active ingredient into the bloodstream at the same rate and extent. Key concepts include the measurement of pharmacokinetic parameters such as Cmax (maximum concentration) and AUC (area under the curve) to demonstrate therapeutic equivalence. Regulatory agencies require bioequivalence studies to confirm that generic drugs provide the same efficacy and safety as their brand-name counterparts.
Bioavailability vs Bioequivalence: Core Differences
Bioavailability measures the rate and extent to which an active drug ingredient is absorbed into the systemic circulation, directly impacting its therapeutic effect. Bioequivalence compares two drug products to ensure they release the active ingredient at similar rates and extents, confirming interchangeable efficacy and safety. Understanding these core differences is crucial for drug development, regulatory approval, and clinical substitution of generic medications.
Factors Influencing Bioavailability in Drugs
Factors influencing bioavailability in drugs include the drug's formulation, the route of administration, and the patient's physiological conditions such as gastrointestinal pH, enzyme activity, and blood flow. Drug solubility and stability, first-pass metabolism, and interactions with food or other medications also significantly impact the extent and rate of absorption into systemic circulation. Optimizing these factors enhances therapeutic efficacy and ensures consistent plasma drug concentrations.
Methods to Assess Bioequivalence in Pharmaceuticals
Methods to assess bioequivalence in pharmaceuticals primarily include pharmacokinetic studies that measure the rate and extent of drug absorption by comparing parameters like Cmax and AUC between test and reference formulations. In vivo studies using healthy volunteers under controlled conditions are standard to ensure drug products deliver comparable therapeutic effects. Advanced statistical analysis confirms equivalence within regulatory limits, ensuring safety and efficacy in generic drug approval.
Regulatory Guidelines on Bioavailability and Bioequivalence
Regulatory guidelines on bioavailability and bioequivalence are established by agencies such as the FDA and EMA to ensure generic drugs meet safety and efficacy standards compared to original formulations. Bioavailability assessments measure the rate and extent of active ingredient absorption, while bioequivalence studies compare pharmacokinetic parameters like Cmax and AUC between test and reference products. Compliance with these guidelines is critical for drug approval, enabling therapeutic interchangeability and maintaining public health.
Role of Bioavailability in Drug Development
Bioavailability plays a crucial role in drug development by determining the rate and extent to which the active pharmaceutical ingredient becomes available at the site of action, directly impacting therapeutic efficacy. Optimizing bioavailability ensures that the drug dosage form delivers consistent and predictable plasma concentration levels, influencing absorption, distribution, metabolism, and excretion processes. Accurate assessment of bioavailability facilitates formulation adjustments, enhances patient outcomes, and supports regulatory approval through detailed pharmacokinetic profiling.
Why Bioequivalence Matters in Generic Medicines
Bioequivalence ensures that generic medicines deliver the same active ingredient in the same concentration and rate as their branded counterparts, guaranteeing therapeutic effectiveness and patient safety. Regulatory agencies, such as the FDA and EMA, require bioequivalence studies to approve generics, preventing substandard or ineffective products from entering the market. Maintaining bioequivalence in generics promotes confidence in medication substitution, supports healthcare cost reduction, and sustains treatment outcomes.
Clinical Implications of Bioavailability and Bioequivalence
Bioavailability measures the extent and rate at which an active drug ingredient is absorbed and becomes available at the site of action, critically impacting drug efficacy and dosing accuracy. Bioequivalence ensures that two pharmaceutical products have comparable bioavailability, which is essential when substituting generic drugs for brand-name counterparts to maintain therapeutic consistency. Understanding these clinical implications helps healthcare professionals optimize treatment regimens and ensure patient safety during medication transitions.
Future Directions in Enhancing Bioavailability and Bioequivalence
Future efforts in enhancing bioavailability and bioequivalence will leverage advanced drug delivery systems such as nanoparticles, liposomes, and biodegradable polymers to improve therapeutic efficacy. Innovations in predictive modeling and in vitro-in vivo correlation (IVIVC) techniques will streamline formulation optimization and regulatory approval processes. Personalized medicine approaches integrating pharmacogenomics will further tailor bioavailability profiles to individual patient needs, maximizing drug safety and effectiveness.
Bioavailability vs Bioequivalence Infographic
 difterm.com
difterm.com