Eutrophication occurs when excess nutrients, such as nitrogen and phosphorus, enter aquatic ecosystems, leading to excessive algae growth that depletes oxygen and harms aquatic life. Bioaccumulation refers to the buildup of toxic substances, like heavy metals or pesticides, in organisms over time, often increasing in concentration up the food chain. Both processes disrupt ecosystem balance but affect the environment through different mechanisms--nutrient overload versus toxin accumulation.
Table of Comparison
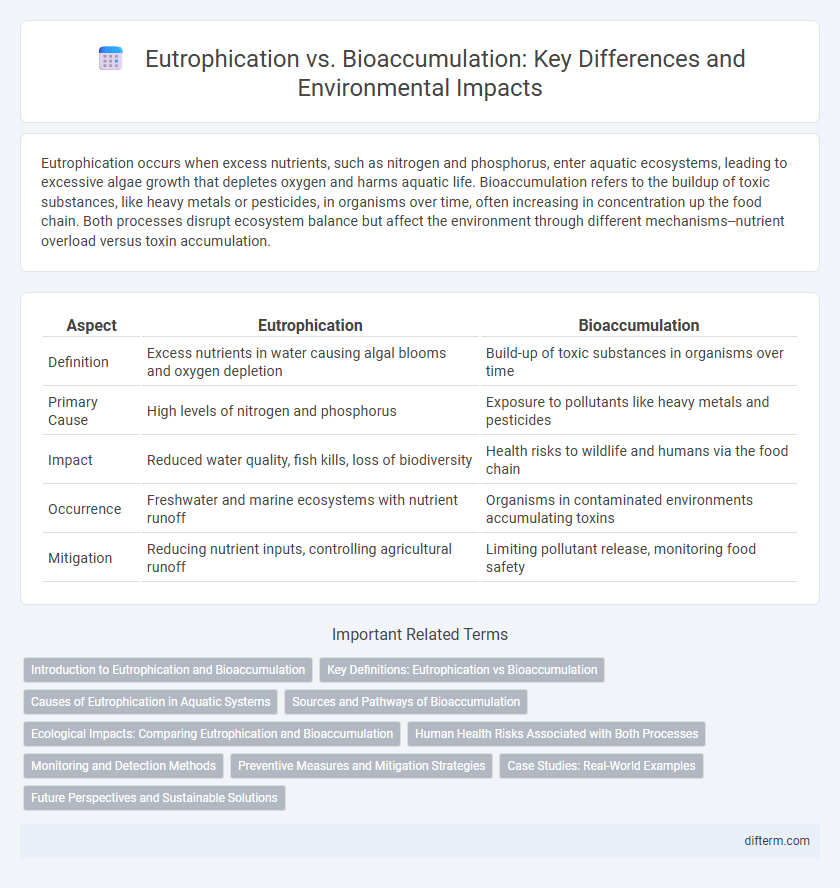
| Aspect | Eutrophication | Bioaccumulation |
|---|---|---|
| Definition | Excess nutrients in water causing algal blooms and oxygen depletion | Build-up of toxic substances in organisms over time |
| Primary Cause | High levels of nitrogen and phosphorus | Exposure to pollutants like heavy metals and pesticides |
| Impact | Reduced water quality, fish kills, loss of biodiversity | Health risks to wildlife and humans via the food chain |
| Occurrence | Freshwater and marine ecosystems with nutrient runoff | Organisms in contaminated environments accumulating toxins |
| Mitigation | Reducing nutrient inputs, controlling agricultural runoff | Limiting pollutant release, monitoring food safety |
Introduction to Eutrophication and Bioaccumulation
Eutrophication is a process where water bodies become enriched with nutrients, primarily nitrogen and phosphorus, leading to excessive growth of algae and aquatic plants. This nutrient overload causes oxygen depletion, harming aquatic life and disrupting ecosystems. Bioaccumulation refers to the gradual buildup of toxins, such as heavy metals and pesticides, in organisms, which can magnify through the food chain and pose significant risks to wildlife and human health.
Key Definitions: Eutrophication vs Bioaccumulation
Eutrophication is the process where water bodies become enriched with excess nutrients, primarily nitrogen and phosphorus, leading to excessive algal growth and oxygen depletion. Bioaccumulation refers to the gradual accumulation of toxins, such as heavy metals or pesticides, in the tissues of organisms over time. Both phenomena disrupt aquatic ecosystems but differ in mechanisms and environmental impacts.
Causes of Eutrophication in Aquatic Systems
Eutrophication in aquatic systems is primarily caused by the excessive input of nutrients, particularly nitrogen and phosphorus, from agricultural runoff, wastewater discharge, and industrial effluents. These nutrients stimulate rapid algal blooms that deplete oxygen levels, leading to hypoxic conditions detrimental to aquatic life. Unlike bioaccumulation, which involves the buildup of toxic substances in organisms, eutrophication results from nutrient enrichment disrupting ecosystem balance.
Sources and Pathways of Bioaccumulation
Bioaccumulation occurs when toxic substances such as heavy metals, pesticides, and persistent organic pollutants enter ecosystems through industrial discharge, agricultural runoff, and atmospheric deposition. These contaminants accumulate in aquatic organisms via water, sediment, and food chains, progressively increasing in concentration at higher trophic levels. The primary pathways include direct absorption from water, ingestion of contaminated prey, and sediment contact in aquatic habitats.
Ecological Impacts: Comparing Eutrophication and Bioaccumulation
Eutrophication causes nutrient over-enrichment in aquatic ecosystems, leading to harmful algal blooms, oxygen depletion, and loss of biodiversity. Bioaccumulation involves the gradual buildup of toxic substances like heavy metals and pesticides in organisms, resulting in reproductive failures and biomagnification through food chains. Both processes disrupt ecosystem stability but eutrophication primarily affects water quality and oxygen levels, while bioaccumulation poses long-term toxicity risks to wildlife and humans.
Human Health Risks Associated with Both Processes
Eutrophication leads to harmful algal blooms that produce toxins threatening human health through contaminated water and seafood consumption, causing illnesses such as liver damage and neurological effects. Bioaccumulation concentrates hazardous substances like heavy metals and persistent organic pollutants in aquatic organisms, posing significant health risks including cancer, immune system damage, and developmental disorders when these organisms enter the human food chain. Both processes amplify exposure to toxic compounds, highlighting the critical need for monitoring and mitigating environmental pollution to safeguard public health.
Monitoring and Detection Methods
Monitoring eutrophication relies heavily on water quality assessments such as measuring nutrient concentrations (nitrogen and phosphorus), chlorophyll-a levels, and dissolved oxygen to detect algal blooms and hypoxic conditions. Bioaccumulation detection involves analyzing tissue samples from aquatic organisms for contaminants like heavy metals, pesticides, and persistent organic pollutants using advanced techniques such as gas chromatography-mass spectrometry (GC-MS) and inductively coupled plasma mass spectrometry (ICP-MS). Remote sensing and in-situ sensors play a crucial role in real-time monitoring, enabling early detection and management of both eutrophication and bioaccumulation in aquatic ecosystems.
Preventive Measures and Mitigation Strategies
Preventive measures for eutrophication include reducing nutrient runoff by implementing sustainable agricultural practices, such as buffer strips and controlled fertilizer application, while bioaccumulation mitigation focuses on minimizing the release of persistent organic pollutants and heavy metals into ecosystems. Monitoring water quality and enforcing regulations on industrial discharge play crucial roles in controlling contaminant levels and preventing their entry into food chains. Remediation techniques like phytoremediation and sediment removal help restore affected water bodies and reduce toxin buildup in organisms.
Case Studies: Real-World Examples
Eutrophication in Lake Erie has caused massive algal blooms, depleting oxygen levels and leading to fish kills, highlighting nutrient pollution impacts on freshwater ecosystems. The Minamata Bay mercury poisoning exemplifies bioaccumulation, where mercury concentrations magnified through aquatic food chains caused severe neurological damage in human populations. These cases underscore the contrasting mechanisms and ecological consequences of nutrient over-enrichment versus toxic substance accumulation in the environment.
Future Perspectives and Sustainable Solutions
Advancements in bioremediation and nutrient management technologies promise to mitigate eutrophication by reducing nitrogen and phosphorus runoff into aquatic ecosystems. Emerging research on biomagnification highlights the need for stricter regulations on heavy metal emissions to prevent bioaccumulation in food chains. Sustainable solutions emphasize integrated watershed management and green infrastructure to restore ecosystem balance and protect biodiversity long-term.
Eutrophication vs Bioaccumulation Infographic
 difterm.com
difterm.com