Autophagy is a cellular process that recycles damaged organelles and proteins, promoting cell survival under stress conditions, while apoptosis is programmed cell death essential for removing damaged or harmful cells. Both mechanisms maintain cellular homeostasis, but autophagy primarily supports cell repair, whereas apoptosis eliminates cells beyond repair. Understanding the balance between autophagy and apoptosis is crucial for developing therapies targeting cancer, neurodegenerative diseases, and aging.
Table of Comparison
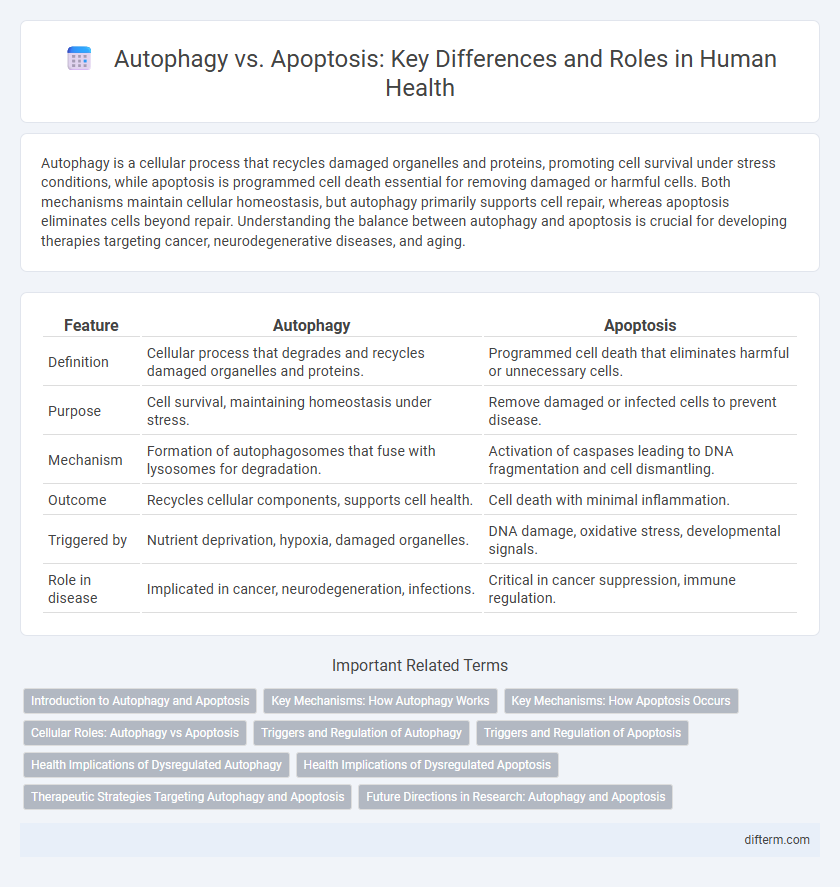
| Feature | Autophagy | Apoptosis |
|---|---|---|
| Definition | Cellular process that degrades and recycles damaged organelles and proteins. | Programmed cell death that eliminates harmful or unnecessary cells. |
| Purpose | Cell survival, maintaining homeostasis under stress. | Remove damaged or infected cells to prevent disease. |
| Mechanism | Formation of autophagosomes that fuse with lysosomes for degradation. | Activation of caspases leading to DNA fragmentation and cell dismantling. |
| Outcome | Recycles cellular components, supports cell health. | Cell death with minimal inflammation. |
| Triggered by | Nutrient deprivation, hypoxia, damaged organelles. | DNA damage, oxidative stress, developmental signals. |
| Role in disease | Implicated in cancer, neurodegeneration, infections. | Critical in cancer suppression, immune regulation. |
Introduction to Autophagy and Apoptosis
Autophagy is a cellular process that degrades and recycles damaged organelles and proteins to maintain cellular homeostasis and survival under stress conditions. Apoptosis, also known as programmed cell death, eliminates damaged or unwanted cells through a regulated sequence of molecular events to prevent inflammation and maintain tissue health. Both processes are critical for cellular quality control, with autophagy promoting cell survival and apoptosis facilitating controlled cell death.
Key Mechanisms: How Autophagy Works
Autophagy is a cellular degradation process that involves the formation of double-membrane vesicles called autophagosomes, which engulf damaged organelles and proteins, delivering them to lysosomes for breakdown and recycling. This mechanism maintains cellular homeostasis by removing dysfunctional components and providing metabolic substrates during stress or nutrient deprivation. Key molecular players in autophagy include the ULK1 complex for initiation, Beclin-1 for vesicle nucleation, and LC3 for autophagosome membrane expansion and closure.
Key Mechanisms: How Apoptosis Occurs
Apoptosis occurs through a tightly regulated process involving signaling pathways such as the intrinsic (mitochondrial) and extrinsic (death receptor) pathways, which activate caspases that dismantle cellular components. Key proteins like Bcl-2 family members regulate mitochondrial membrane permeability, leading to cytochrome c release and apoptosome formation. This cascade triggers proteolytic enzymes that systematically degrade the cell, ensuring controlled elimination without inflammation.
Cellular Roles: Autophagy vs Apoptosis
Autophagy is a cellular process that promotes survival by degrading and recycling damaged organelles and proteins to maintain homeostasis under stress conditions. Apoptosis, by contrast, is a programmed cell death mechanism that removes damaged or potentially harmful cells to prevent disease development. Both processes are critical for tissue health and disease prevention but serve distinct roles: autophagy sustains cellular function, whereas apoptosis eliminates dysfunctional cells.
Triggers and Regulation of Autophagy
Autophagy is primarily triggered by nutrient starvation, hypoxia, and cellular stress, activating signaling pathways such as mTOR inhibition and AMPK activation to regulate cellular degradation and recycling processes. In contrast, apoptosis is often initiated by DNA damage, oxidative stress, or death receptor signaling, involving caspase activation for programmed cell death. The tightly controlled regulation of autophagy maintains cellular homeostasis and survival during metabolic stress, differentiating it functionally and mechanistically from apoptosis.
Triggers and Regulation of Apoptosis
Apoptosis is regulated by intrinsic signals such as DNA damage and oxidative stress, as well as extrinsic signals including death ligands like FasL and TNF-alpha binding to their receptors. Key regulators involve the Bcl-2 protein family, which controls mitochondrial outer membrane permeability, and caspases, which execute the cell death program. In contrast, autophagy is primarily triggered by nutrient deprivation and cellular stress to maintain homeostasis through lysosomal degradation.
Health Implications of Dysregulated Autophagy
Dysregulated autophagy disrupts cellular homeostasis, contributing to the development of neurodegenerative diseases such as Parkinson's and Alzheimer's by impairing the clearance of damaged proteins and organelles. In cancer, defective autophagy can promote tumor progression by allowing damaged cells to survive and proliferate unchecked. Targeting autophagy pathways holds potential for therapeutic interventions in metabolic disorders, infections, and aging-related conditions by restoring cellular balance and function.
Health Implications of Dysregulated Apoptosis
Dysregulated apoptosis disrupts cellular homeostasis and contributes to various health disorders, including cancer, autoimmune diseases, and neurodegenerative conditions. Impaired apoptosis allows damaged or dysfunctional cells to survive, leading to abnormal tissue growth or chronic inflammation. Understanding the balance between autophagy and apoptosis is critical for developing therapies that restore proper cell death mechanisms and improve disease outcomes.
Therapeutic Strategies Targeting Autophagy and Apoptosis
Therapeutic strategies targeting autophagy and apoptosis focus on modulating cellular pathways to treat diseases such as cancer, neurodegeneration, and infections. Autophagy inducers like rapamycin enhance cellular cleanup processes, promoting survival and reducing inflammation, while apoptosis modulators such as BH3 mimetics trigger programmed cell death in malignant cells. Combination therapies that balance autophagy and apoptosis offer promising clinical outcomes by restoring cellular homeostasis and enhancing treatment efficacy.
Future Directions in Research: Autophagy and Apoptosis
Emerging research in autophagy and apoptosis is focused on elucidating their intricate molecular pathways to develop targeted therapies for neurodegenerative diseases and cancer. Advances in single-cell sequencing and CRISPR gene editing are enabling precise modulation of these processes to enhance cell survival or induce programmed cell death. Future studies aim to integrate autophagy and apoptosis mechanisms to optimize therapeutic strategies for age-related disorders and immune system regulation.
Autophagy vs Apoptosis Infographic
 difterm.com
difterm.com