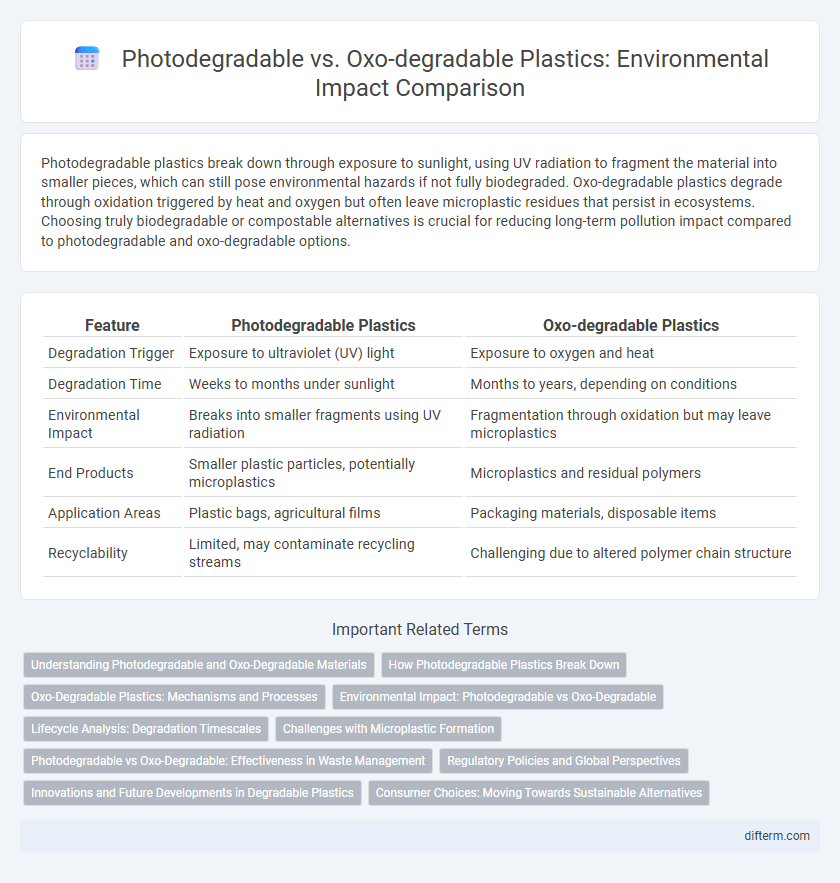
Photodegradable plastics break down through exposure to sunlight, using UV radiation to fragment the material into smaller pieces, which can still pose environmental hazards if not fully biodegraded. Oxo-degradable plastics degrade through oxidation triggered by heat and oxygen but often leave microplastic residues that persist in ecosystems. Choosing truly biodegradable or compostable alternatives is crucial for reducing long-term pollution impact compared to photodegradable and oxo-degradable options.
Table of Comparison
| Feature | Photodegradable Plastics | Oxo-degradable Plastics |
|---|---|---|
| Degradation Trigger | Exposure to ultraviolet (UV) light | Exposure to oxygen and heat |
| Degradation Time | Weeks to months under sunlight | Months to years, depending on conditions |
| Environmental Impact | Breaks into smaller fragments using UV radiation | Fragmentation through oxidation but may leave microplastics |
| End Products | Smaller plastic particles, potentially microplastics | Microplastics and residual polymers |
| Application Areas | Plastic bags, agricultural films | Packaging materials, disposable items |
| Recyclability | Limited, may contaminate recycling streams | Challenging due to altered polymer chain structure |
Understanding Photodegradable and Oxo-Degradable Materials
Photodegradable materials break down through exposure to natural sunlight, using ultraviolet (UV) rays to degrade the polymer chains, which results in faster decomposition compared to conventional plastics. Oxo-degradable materials contain additives like metal salts that accelerate oxidation and fragmentation when exposed to heat, oxygen, or UV light but often leave behind microplastics rather than fully decomposing. Understanding these mechanisms is crucial for assessing the environmental impact and sustainability of using these materials in packaging and waste management.
How Photodegradable Plastics Break Down
Photodegradable plastics break down through exposure to sunlight, specifically ultraviolet (UV) radiation, which triggers a chemical reaction that fragments the polymer chains. This process results in the plastic degrading into smaller, non-toxic molecules like carbon dioxide, water, and biomass, enhancing environmental safety. Unlike oxo-degradable plastics, photodegradable variants do not rely on additives that cause incomplete degradation and microplastic formation.
Oxo-Degradable Plastics: Mechanisms and Processes
Oxo-degradable plastics contain additives such as metal salts that catalyze polymer chain scission through oxidation when exposed to heat, oxygen, or UV light, accelerating their fragmentation into microplastics. The oxidation process involves the formation of free radicals that break down long polymer chains, resulting in brittle and fragmented materials that do not fully biodegrade but fragment into smaller particles. This partial degradation poses environmental concerns as oxo-degradable plastics persist in ecosystems, contributing to microplastic pollution rather than complete mineralization.
Environmental Impact: Photodegradable vs Oxo-Degradable
Photodegradable plastics break down through exposure to sunlight, reducing long-term environmental persistence but can leave microplastic residues that harm marine ecosystems. Oxo-degradable plastics fragment faster with oxygen and heat but do not fully biodegrade, contributing to soil pollution and potentially toxic byproducts. The comprehensive environmental impact of both materials depends on degradation completeness and ecosystem interactions, with photodegradable options generally posing fewer risks than oxo-degradable counterparts.
Lifecycle Analysis: Degradation Timescales
Photodegradable plastics typically break down within months to a few years under sunlight exposure, relying on UV radiation to initiate polymer fragmentation. Oxo-degradable plastics degrade through oxidation catalyzed by additives, often resulting in fragmentation over several months to decades, depending on environmental conditions. Lifecycle analysis reveals photodegradables generally offer faster degradation but may lead to microplastic formation, while oxo-degradables present longer persistence and uncertain end-stage biodegradability.
Challenges with Microplastic Formation
Photodegradable plastics break down through exposure to sunlight but often fragment into microplastics that persist in the environment, posing significant ecological risks. Oxo-degradable plastics rely on additives to accelerate degradation, yet these materials also fragment into microplastics rather than fully mineralizing, contributing to soil and marine pollution. Both types face challenges in preventing microplastic formation, complicating waste management and environmental health efforts.
Photodegradable vs Oxo-Degradable: Effectiveness in Waste Management
Photodegradable plastics break down through exposure to sunlight, utilizing ultraviolet rays to initiate polymer degradation, resulting in faster fragmentation compared to oxo-degradable plastics, which rely on chemical additives to trigger oxidation under heat and oxygen. Research shows photodegradable materials tend to reduce microplastic formation more effectively by fully degrading into environmentally benign compounds, whereas oxo-degradable plastics often leave behind persistent microplastics that complicate waste management. These differences significantly influence landfill accumulation rates, recycling processes, and environmental toxicity, making photodegradable materials more favorable for sustainable waste reduction strategies.
Regulatory Policies and Global Perspectives
Regulatory policies increasingly favor photodegradable materials due to their more predictable environmental degradation pathways compared to oxo-degradable plastics, which fragment into microplastics and pose greater ecological risks. The European Union has banned oxo-degradable plastics under the Single-Use Plastics Directive, citing insufficient evidence of their biodegradability and harmful environmental impact. Global perspectives show divergent approaches, with some countries promoting photodegradable alternatives as part of sustainable waste management strategies, while others still lack comprehensive regulatory frameworks addressing both types.
Innovations and Future Developments in Degradable Plastics
Innovations in degradable plastics are rapidly advancing, with photodegradable materials designed to break down under specific light wavelengths, enabling controlled degradation in natural environments. Emerging research focuses on enhancing the efficacy of oxo-degradable plastics by integrating catalysts that accelerate fragmentation while minimizing microplastic pollution. Future developments aim to create hybrid systems combining photodegradable and oxo-degradable mechanisms, optimizing environmental degradation rates and reducing plastic persistence in ecosystems.
Consumer Choices: Moving Towards Sustainable Alternatives
Photodegradable plastics break down into smaller fragments through exposure to sunlight, while oxo-degradable plastics degrade via oxidation, often leaving behind microplastics that persist in the environment. Consumers increasingly prefer photodegradable options as they pose less risk of long-term pollution and support the shift towards sustainable waste management. Choosing biodegradable and compostable materials further reduces ecological impact, aligning consumer behavior with global environmental goals.
photodegradable vs oxo-degradable Infographic
 difterm.com
difterm.com